Inside The FDA's New Guidance On Mammograms
October is Breast Cancer Awareness Month, which aims to help people understand the risks and seek tests for breast cancer (via the New Mexico Department of Health). Women are the most at risk of facing a breast cancer diagnosis, though it can impact men too. However, women need to be especially vigilant.
If you are a woman, 50 years or older, have a family history of cancer, or have had changes in your BRCA1 or BRCA2 genes, you may be at risk of experiencing a breast cancer diagnosis. However, there are ways to lower your risk. For instance, some doctors recommend keeping a healthy lifestyle that includes working out, avoiding or limiting alcohol consumption, and making sure you check with your primary care provider if you are taking birth control or hormone replacement therapy.
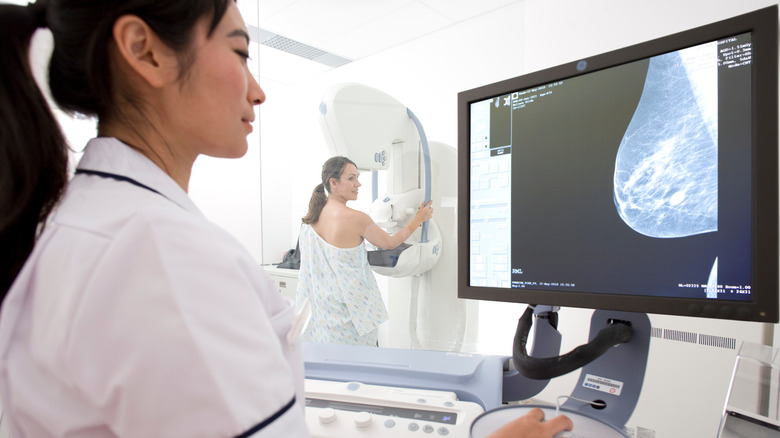
Another way to stay on top of your breast health is to book breast cancer screenings, or mammograms, in the recommended timeframe depending on your age (per the Centers for Disease Control and Prevention). Now, the FDA is implementing new mammogram regulations that will help women with different breast types.
The FDA is putting new guidelines in place to help women with dense breasts
Mammograms can be beneficial in getting a breast cancer diagnosis as early as possible, Breastcancer.org reports. When caught early, treatment is more manageable and the chance of survival is higher. However, the process of this form of cancer screening is far from perfect.
For a disease that affects one in eight women, according to the American Cancer Society, it's important to have the best tools to detect cancer. However, the mammogram process has struggled to diagnose women with more dense breasts. Now, however, the U.S. Food & Drug Administration is issuing new regulations to help these women.
The American Cancer Society defines dense breast as one that has more tissue than fat, which makes it more difficult when locating tumors in a mammogram screening since the tissue appears the same as tumors in the image. Now, the FDA is planning on providing more information to patients while also having radiologists place patients in one of four breast density categories.
Representative Rosa DeLauro of Connecticut explained what this means for women moving forward. In her statement, she said, "After three years, I am pleased that the FDA has finally communicated that they believe they will be able to roll out a rule requiring providers to notify women of whether they have dense breasts, what this means for their risk of breast cancer, and the importance of speaking with a health care provider about breast density." This process could begin to occur in doctors' offices across the country by the end of the year or early 2023, according to the FDA.